CTC Story
Application
 >
CTC Story
>
Application
>
CTC Story
>
Application
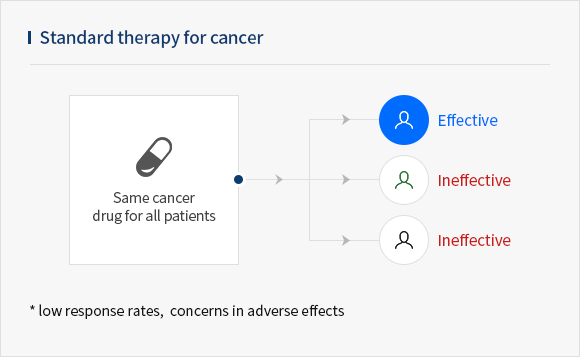
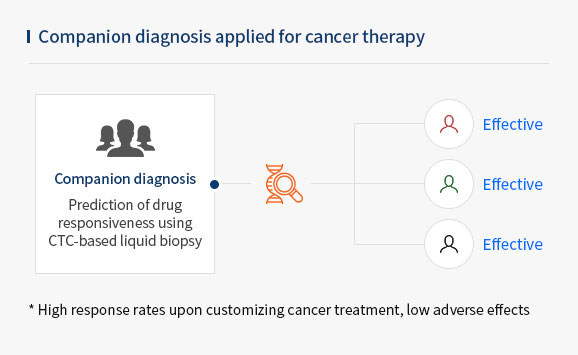
- A circulating tumor cell (CTC) is a cell that has shed into the vasculature or lymphatics from a primary tumor and is carried around the body in the blood circulation. CTC is a potentially useful marker in early diagnosis and monitoring therapeutic effects for patients with malignant tumors.
- CytoGen's CTC liquid biopsy platform isolates naive CTCs in live state and can be used as a companion diagnostic(CDx) for cancer treatment to increase treatment option.
The CTC profiling achieved through our system ultimately contributes to increasing the effectiveness of cancer treatment by selecting optimal drugs for different, and by monitoring cancer prognosis.
Global Liquid Biopsy Network
The collective CTC data via global networks are processed and accumulated as big data.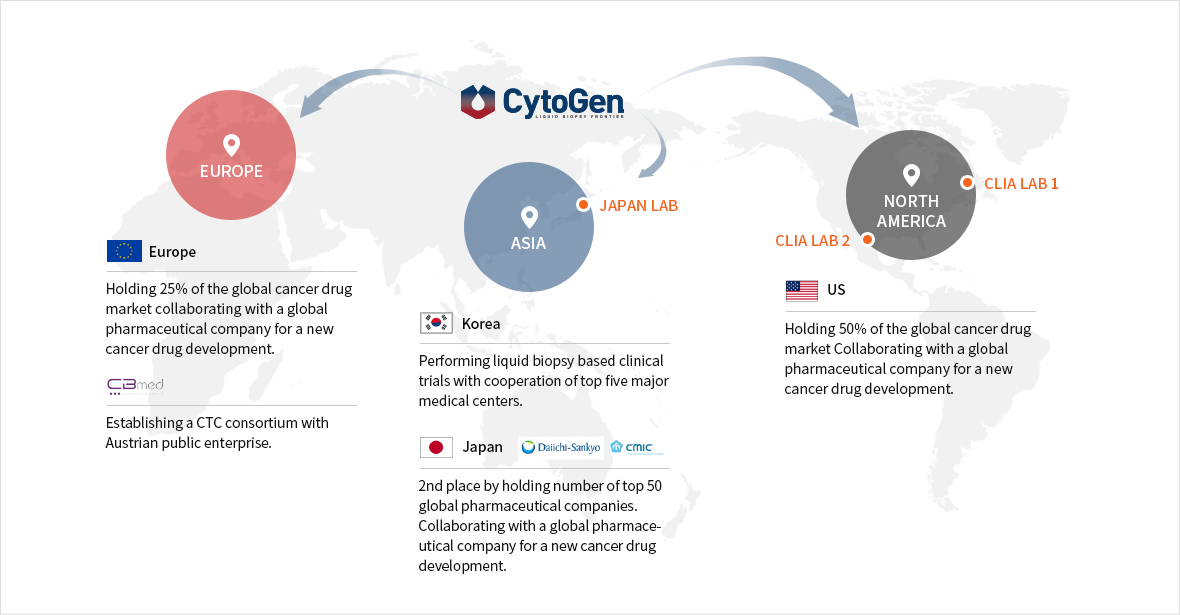
CytoGen's Liquid Biopsy Platform
CTC-based liquid biopsy platform is applicable to any stages of cancer drug development procedures.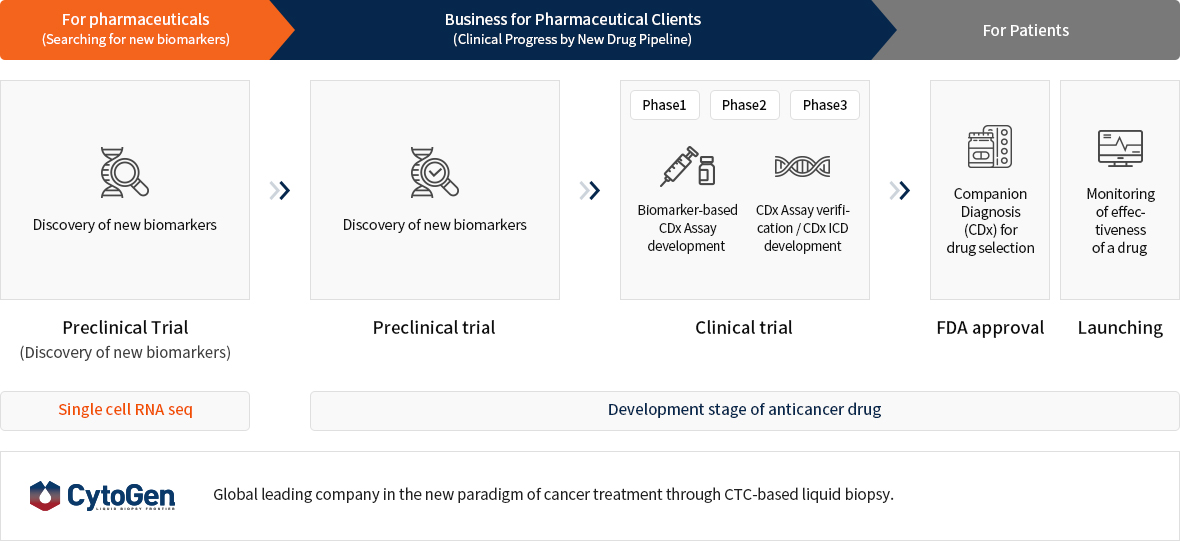
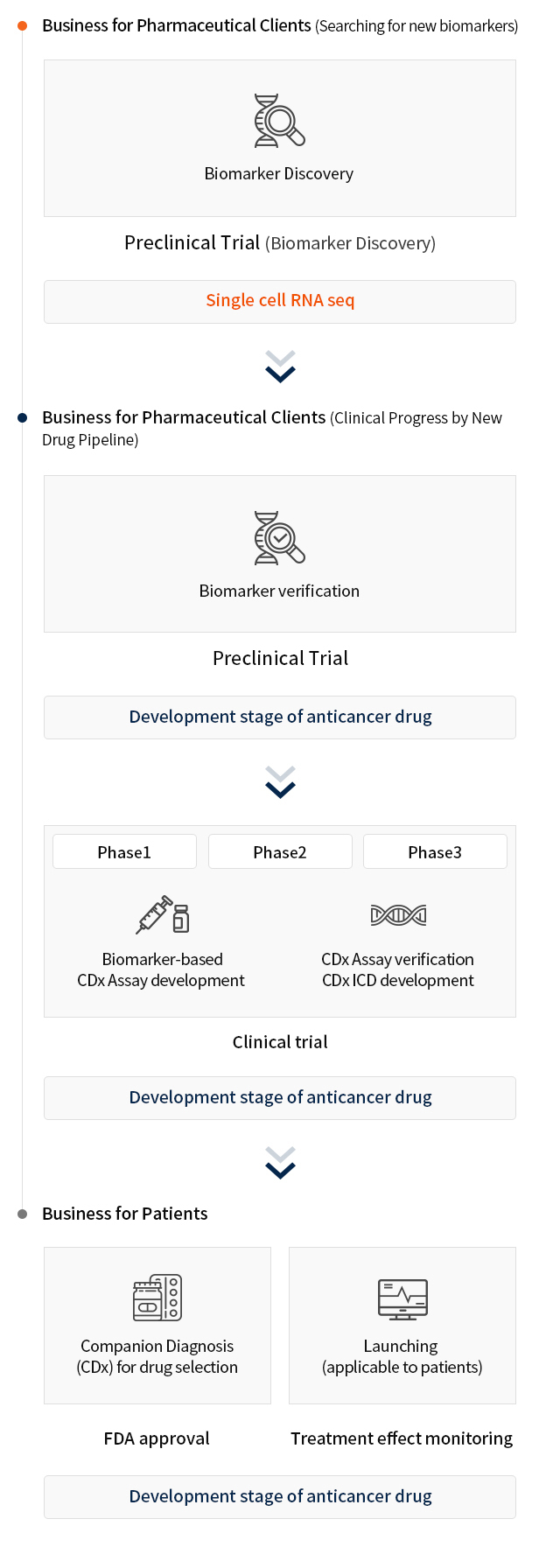
Clinical Applications using CytoGen’s platform
Characterization of CTCs with specific cancer type biomarkers is available for clinical application-

Lung Cancer
-

Pancreas Cancer
-
Bile duct Cancer
-

Prostate Cancer
-

Gastric Cancer
-

Head & Neck Cancer
-

Breast Cancer
-

Colon Cancer
-

Brain Cancer
-

Esophageal Cancer
-

Liver Cancer
-

Sarcoma
-
Ovarian Cancer



